.
Review Process
General Policies and Procedures
Authors submit their manuscripts electronically via Scripture to the Journal of Regenerative Science (JRS). Each manuscript is reviewed by the Journal of Regenerative Science (JRS) staff for relevancy to the individual journal. Should a question arise, the editorial coordinator or the production editor will contact the editor in chief (or an appropriate editor), who then decides whether the manuscript should be editorially rejected owing to scope or retained for review by the journal to which it was submitted. If retained, the manuscript is assigned to an editor, who in turn chooses one or more editorial board members or ad hoc reviewers to review it.
The peer review is a Double-Blind Peer Review where the reviewers do not know the identity of the authors and authors do not know the reviewers
Only Invited Guest Editorial are exempt from the peer-review process. Remaining all article types undergo a peer review process as below
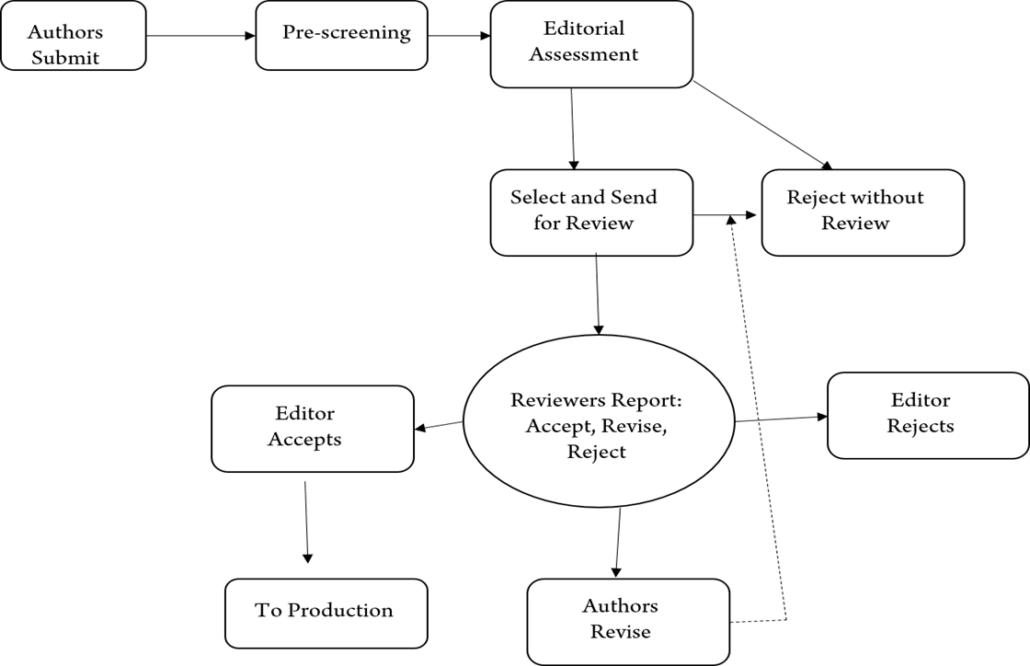
Instructions to Reviewers
General Policies and Procedures
Authors submit their manuscripts electronically via Scripture to the Journal of Regenerative Science (JRS). Each manuscript is reviewed by the Journal of Regenerative Science (JRS) staff for relevancy to the individual journal. Should a question arise, the editorial coordinator or the production editor will contact the editor in chief (or an appropriate editor), who then decides whether the manuscript should be editorially rejected owing to scope, or retained for review by the journal to which it was submitted. If retained, the manuscript is assigned to an editor, who in turn chooses one or more editorial board members or ad hoc reviewers to do the double-blind review.
Review Steps
1.Notify the submission’s editor as to whether you will undertake the review.
Response Will do the review Unable to do the review
2.Click on file names to download and review (on screen or by printing) the
files associated with this submission.
Submission will be made available, if and when the reviewer agrees to undertake
a review
3.Declare whether or not you have competing interests with regard to this
research (see CI POLICY).
4.Click on the icon to enter (or paste) your review of this submission.
5.In addition, you can upload files for the editor and/or author to consult.
- Select a recommendation and submit the review to complete the process.
You must enter a review or upload a file before selecting a recommendation.
If you have either a time problem or a conflict of interest, contact the editor for instructions. He/she may extend your deadline or cancel the review assignment as appropriate. If your cursory examination reveals that the manuscript does not fit within the scope of the journal, indicate that in the Confidential Comments to the Editor section of the review form.
Do not discuss the paper with its authors either during or after the review process. Although it may seem natural and reasonable to discuss points of difficulty or disagreement directly with an author, especially if you are generally in favor of publication and do not mind revealing your identity, this practice is prohibited because the other reviewers and the editor may have different opinions, and the author may be misled by having “cleared things up” with the reviewer who contacted him/her directly.
The manuscript provided to you for review is a privileged document. Please protect it from any form of exploitation. Do not cite a manuscript or refer to the work it describes before it has been published and do not use the information that it contains for the advancement of your own research or in discussions with colleagues.
In your comments intended for the author, do not make statements about the acceptability of a paper (see the next paragraph); suggested revisions should be stated as such and not expressed as conditions of acceptance.
Organize your review into three parts: Introductory paragraph, Major Comments, Minor Comments
- The introductory paragraph summarizes the major findings of the article, gives your overall impression of the paper, and highlights the major shortcomings. This paragraph should be followed by specific, numbered comments, which, if appropriate, may be subdivided into
- Major Points – which may include errors in documentation or interpretation of data, lack of data, missing information or more information needed like follow-up or more up-to-date review of the literature.
- Minor points – like details of figures, providing better figures, providing some additional clinical details, etc.
(The numbering facilitates both the editor’s letter to the author and evaluation of the author’s rebuttal.) Criticism should be presented dispassionately; offensive remarks are not acceptable.
Confidential remarks directed to the editor should be entered in the box so labeled. Advise the editor of your recommendation for acceptance, modification, or rejection by making the appropriate selection in the dropdown menu. The final decision regarding modification, acceptance, or rejection of a manuscript rests solely with the editor, so do not state your recommendation in the portion of the review that will be sent to the author.
After completing your review, click the “Submit Review” button. You may want to save a copy of your review offline for your records. After successful completion of your review, it will be saved in your Past Reviews folder in Scripture.
The Review
Adopt a positive, impartial, but critical attitude toward the manuscript under review, with the aim of promoting effective, accurate, and relevant scientific communication.
Please consider the following aspects when reviewing a manuscript:
- Significance to the target scientific community
- Originality
- Appropriateness of the approach or experimental design
- Adherence to correct scientific nomenclature
- Appropriate literature citations
- The soundness of conclusions and interpretation
- Relevance of discussion
- Organization
- Adherence to the Instructions to Authors
- Adequacy of title and abstract
- Appropriateness of figures and tables
- Appropriateness of supplemental material intended for posting (if applicable)
- Length
- Whether it clearly describes the Clinical Relevance of the article
You are not required to correct deficiencies of style, syntax, or grammar, but any help you can give in clarifying meaning will be appreciated. In particular, point out the use of scientific jargon, misspellings of chemical names, use of outmoded terminology or incorrect genetic nomenclature, and use of misspelled, incorrect, or outdated scientific names of organisms.
Your criticisms, arguments, and suggestions concerning the paper will be most useful to the editor and to the author if they are carefully documented. Do not make dogmatic, dismissive statements, particularly about the novelty of the work. Substantiate your statements. Reviewer’s recommendations are gratefully received by the editor; however, since editorial decisions are usually based on evaluations derived from several sources, reviewers should not expect the editor to honor every recommendation. You will be asked to suggest acceptability as noted on the specific review form (e.g., accept; accept with revision; reject; modify, re-review required).
- Very few papers qualify for an immediate, unconditional acceptance.
- There are many reasons to reject a paper. In general, if there are serious flaws in the clinical report, incorrect interpretation of data, extensive plagiarism, or any organizational or English usage flaws that prevent critical review of the manuscript, then recommend that the manuscript be rejected.
- If you feel that the deficiencies can be corrected within a reasonable period of time, then recommend modification (e.g., modification; accept with revision; or modify – re-review required, if the revisions are extensive enough to warrant a second review).
Journal of Regenerative Science (JRS) Publication Policies; Ethics
Although the staff at the Journal of Regenerative Science (JRS) Journals Department and the journal editors may be able to note a breach of publication policy or ethical conduct after publication, we rely heavily on the reviewers to detect such problems before publication. Journal of Regenerative Science (JRS) publication policies are described in the Instructions to Authors, which are available online. Some of the items for which you should be alert include:
- Plagiarism – Plagiarism is not limited to the Results and Discussion sections; it can involve any part of the manuscript, including figures and tables, in which material is copied from another publication without attestation, reference, or permission. Note that wording does not have to be exact to be copyright infringement; the use of very similar words in almost the same sequence can also be infringement. Data themselves are not copyrightable, but their presentation is.
- Missing or incomplete attestation – Authors must give appropriate credit to ideas, concepts, and data that have been published previously. This is accomplished by the inclusion of references. Missing, incomplete, or incorrect references must be brought to the editor’s attention.
- Dual submission and/or publication – Be wary of attempts to submit/publish similar material more than once. This is often difficult to detect “before the fact,” but checking literature citations, as well as having a critical eye, is helpful.
- Conflicts of interest – If you are aware of any commercial affiliations, consultancies, stock or equity interests, or patent-licensing arrangements on the part of the authors, bring them to the attention of the editor.
Note that similar conflicts of interest on your part must also be brought to the attention of the editor, who may, at his discretion, subsequently cancel your invitation to review the manuscript. If one of the manuscript authors is at your institution, there could be a perceived conflict of interest, and you should immediately contact the editor so that another individual can be invited to review the manuscript in your place.
In summary, you must communicate suspicions of policy or ethics problems directly to the editor, who in turn will contact the editor in chief. Under no circumstance should you contact the author directly. Journal of Regenerative Science (JRS) has policies for investigation and resolution of such problems and these must be followed.
Editorial and Ethics Policies
Journal Ethics
Journal/Association are committed to meeting and upholding standards of ethical behavior at all stages of the publication process. We follow closely the industry associations, such as the Committee on Publication Ethics (COPE), International Committee of Medical Journal Editors (ICMJE) and World Association of Medical Editors (WAME), that set standards and provide guidelines for best practices in order to meet these requirements.
Editorial Policies
We expect the highest ethical standards from their authors, reviewers and editors when conducting research, submitting papers and throughout the peer-review process.
Redundant or duplicate publication
Duplicate or redundant publication is a publication that overlaps substantially with one already published, in press, or in an electronic media submission. (International Committee of Medical Journal Editors.
Duplicate or redundant submission is the same manuscript (or the same data) that is submitted to different journals at the same time. International copyright laws, ethical conduct, and cost-effective use of resources require that readers can be assured that what they are reading is original. (International Committee of Medical Journal Editors. http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/overlapping-publications.html Submitted manuscripts should not have been published or currently submitted elsewhere. Duplicate publication is a violation of the APA code of ethics (APA Publication Manual, 2010) and will be grounds for prompt rejection of the submitted manuscript. If the editor was not aware of the violation and the article has been published, a notice of duplicate submission and the ethical violation will be published.
Retraction policy
Journal of Regenerative Science abide by COPE Retraction Guidelines.
COPE Retraction Guidelines: https://publicationethics.org/retraction-guidelines
Conflicts of interest
At the point of submission, policy requires that each author reveal any financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated – including pertinent commercial or other sources of funding for the individual author(s) or for the associated department(s) or organization(s), personal relationships, or direct academic competition.
If the manuscript is accepted, Conflict of Interest information will be communicated in a published statement.
Permissions to reproduce previously published material
Permission is required to reproduce material (such as illustrations) from the copyright holder. Articles cannot be published without these permissions.
Patient consent forms
The protection of a patient’s right to privacy is essential. Please collect and keep copies of patients’ consent forms on which patients or other subjects of your experiments clearly grant permission for the publication of photographs or other material that might identify them. If the consent form for your research did not specifically include this, please obtain it or remove the identifying material.
A statement to the effect that such consent had been obtained must be included in the ‘Methods’ section of your paper. If necessary, the Editors may request a copy of any consent forms.
Ethics committee approval
All articles dealing with original human or animal data must include a statement on ethics approval at the beginning of the Methods section. This paragraph must contain the following information: the name and address of the ethics committee responsible; the protocol number that was attributed by this ethics committee; and the date of approval by the ethics committee.
The paragraph could read, for example:
“Ethical approval for this study (Ethical Committee N° NAC 207) was provided by the Ethical Committee NAC of Geneva University Hospitals, Geneva, on 12 February 2007.”
In addition, and as stated above, for studies conducted on human participants you must state clearly that you obtained written informed consent from the study participants; please also look at the latest version of the Declaration of Helsinki. Similarly, for experiments involving animals you must state the care of animal and licensing guidelines under which the study was performed and report these in accordance with the ARRIVE (Animals in Research: Reporting In Vivo Experiments) statement. If ethics clearance was not necessary, or if there was any deviation from these standard ethical requests, please state why it was not required. Please note that the editors may ask you to provide evidence of ethical approval. If you have approval from a National Drug Agency (or similar) please state this and provide details, this can be particularly useful when discussing the use of unlicensed drugs.
Plagiarism
Articles published at JRS, evaluate submissions on the understanding that they are the original work of the author(s). Re-use of text, data, figures, or images without appropriate acknowledgment or permission is considered plagiarism, as is the paraphrasing of text, concepts, and ideas. All allegations of plagiarism are investigated in accordance with COPE guidelines detailed at https://publicationethics.org/files/u7140/plagiarism%20A.pdf
